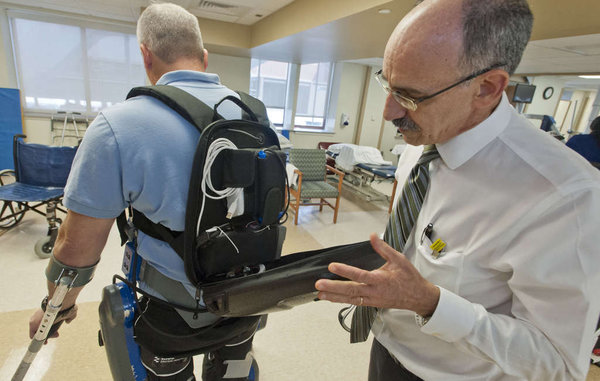
With a hoist and a sudden mechanical groan, Dan Webb, 44, stands up in the rehabilitation gym at MossRehab in Elkins Park, grinning from ear to ear.
You might say standing is not such a big deal, unless, like Webb, who fell from a tree stand while bowhunting in 2010 and suffered incomplete paralysis of the lower half of his body, you’ve spent a year of your life in a wheelchair.
In his case, it’s a very big deal indeed.
And Webb, of Warminster – who has been working with exoskeleton technology for two years at MossRehab – can do more than stand. With a whir of motors and a flash of crutches, he turns and walks across the gym floor and back again.
“What I love the most,” he says, “is looking at people eye to eye.”
What has changed Webb’s life for the better is the ReWalk Rehabilitation, a computerized exoskeleton made by Israel’s ReWalk Robotics. Designed to help give mobility to people with lower-body paralysis, it received Food and Drug Administration approval last month, becoming the first device of its kind certified for home use. MossRehab led testing efforts of the device in the United States.
Exoskeletons provide multiple benefits, says Scott DiDonato of the Plymouth Meeting-based ECRI Institute, which evaluates medical devices. “Studies show that patients heal faster when they are standing upright, internal organs work better, and people use muscles that can otherwise atrophy while seated.”
About 1.3 million people in the U.S. are living with a spinal cord injury, according to the Christopher Reeve Foundation. And those injuries are on the rise, the leading cause being falls among the elderly, according to a Johns Hopkins study.
The ReWalk exoskeleton includes a wearable brace support, a computerized control system, and motion sensors on hips and knees that allow independently controlled walking while mimicking the natural walking gait of an able-bodied person. The 51-pound system is operated wirelessly through a smart watch; a pair of crutches act as “transmitters” to help individuals with partial or no sensation judge the location and position of their legs and feet.
The ReWalk Personal System available for home use, ReWalk-P, is a bit more streamlined than those employed in rehab facilities and can be customized. In general, people who use a ReWalk must be between 5-foot-3 and 6-foot-1 and weigh no more than 220 pounds.
Under the new FDA approval, ReWalk-P is approved for home use only for those with lower spinal injuries (in the T7 (thoracic) vertebrae through the L5 (lumbar) vertebrae). For those with mid-spine injuries, it remains approved for use only in rehabilitation hospitals.
ReWalk-P costs $69,500 and is not yet covered by insurance, although Alberto Esquenazi, the John Otto Haas Chair of Physical Medicine and Rehabilitation at MossRehab, says that as it becomes adopted more widely, insurance may begin to cover it and the price may drop. Company officials say they are in talks with insurers.
Before using a ReWalk, patients must be evaluated. They should be able to stand using an assistive standing device and support crutches or a walker. They should not use it if they have severe spasticity (convulsions in the legs), unhealed limb fractures, or such problems as infections, circulatory conditions, heart or lung conditions, or pressure sores. Patients should also have a DEXA scan to see if their bone density is adequate, Esquenazi says.
Esquenazi, who completed the clinical trial that led to FDA approval, says it took about 24 training sessions for the 16 participants in his study to gain control of the ReWalk, although he believes training now can be done in fewer sessions. His research looked at safety, the ability of participants to use the system, walking velocity, distance walked, and physiological changes related to heart, lung, and bladder function.
“Results showed that it was helpful in reducing spasticity, improving bladder and bowel function, and helping skin issues, because the skin has less chance of breaking down if a person isn’t sitting all the time,” says Esquenazi, who has no financial relationship with the company. “We also think we’re going to see a difference in bone density, since someone sitting all day has very little weight-bearing exercise.”
“ReWalk does not regenerate the spinal cord; there is no good way to do that yet,” Esquenazi says. “But when there is, there will be patients who are prepared for it, with good walking skills and good muscle strength.”
To prevent falls, the ReWalk uses motion sensors and an algorithm that makes it move forward on first one leg, then the next. Other systems, such as the Ekso Bionics exoskeleton, which is powered individually on each side, may eventually help patients with MS, stroke, or traumatic brain injuries, Esquenazi says.
Webb, a former owner of a construction company, had to give up his business after his fall. After he demonstrated the technology at last year’s American Israel Public Affairs Committee meeting in Washington – where he met the Israeli inventor of the ReWalk, Amit Goffer, who became quadriplegic after a 1997 ATV accident – several participants at the conference mounted a private campaign to raise money for Webb to receive a ReWalk-P.
The idea of having his own exoskeleton sends Webb, a married father of two daughters, over the moon. Using a personal exoskeleton, he will be able to drink standing up and even wash dishes at the sink, then turn around and plop down to dinner.
“Sitting in the wheelchair, my two daughters forget how tall I am. I want to go to my daughters’ sporting events, and walk down to talk to the coaches. And I’m looking forward to the daddy-daughter dances.”
“I want to fit in.”
Source: Philly.com
July 21, 2014
By Ilene Raymond Rush
http://articles.philly.com/2014-07-21/news/51786294_1_alberto-esquenazi-mossrehab-paralysis










